Improve Maternal Health Outcomes Committee
The following are projects initiated and supported by the Utah Women and Newborns Quality Collaborative (UWNQC) Improve Maternal Health Outcomes Committee.
Refugee Resource Guide (Salt Lake City) in 5 languages.
Each version has the English version on the second page of the PDF.
Refugee Wellness Guide for Asian Association of Utah (AAU).
Each version of the 4 languages has the English version on the second page of the PDF.
Refugee Wellness Guide for International Rescue Committee (IRC).
Each version of the 4 languages has the English version on the second page of the PDF.
Refugee Wellness Guide for Catholic Community Services (CCS).
Each version of the 4 languages has the English version on the second page of the PDF.
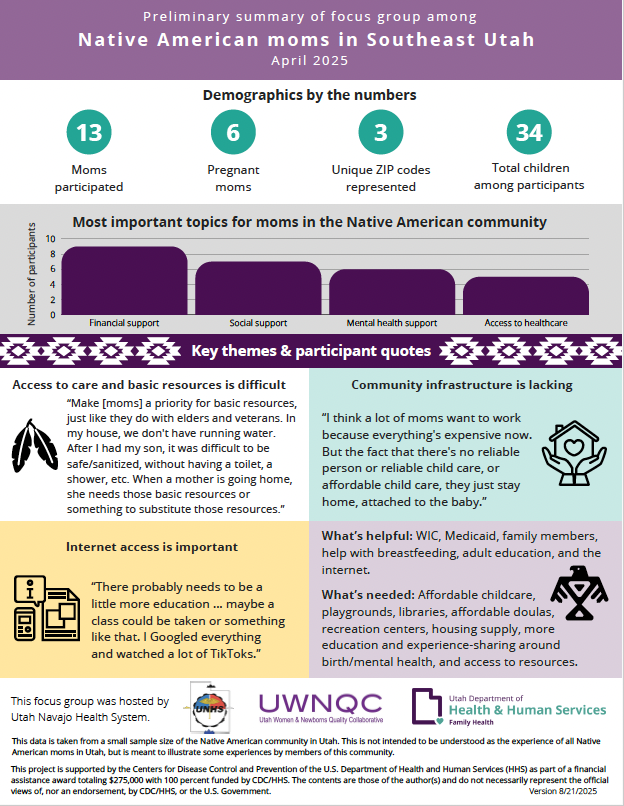
Maternal focus group 2025
San Juan County
Utah Navajo Health System (UNHS) lead a focus group to listen to what moms in San Juan county think would improve maternal health outcomes in the area. The focus group was held at the hogan at the Monument Valley High School.
UWNQC produced an infographic representation of what was shared in the focus group, and overarching topics. UWNQC partners with UNHS on quality improvement work to respond to and aim to improve issues that were discussed in the focus group.
Mama's Village
In April 2025, Utah Women and Newborns Quality Collaborative partnered with community organizations Comunidad Materna, Utah Pacific Islander Health Coalition, and Project Success Coalition to organize a multilingual, multicultural community event for moms and women to gather, connect, and learn about local health resources in Utah.
- 250+ individuals attended.
- 35 organizations participated.
- 104 vaccines were given by the Salt Lake County Health Department.
- 88% of new and expecting moms said they made a meaningful connection with someone at the event.
- 69% of new and expecting moms reported that following the event they felt like it was easier to talk about their mental health.
- Presentations included safe sleep, maternal mental health, mindfulness, babywearing, ZUMBA, African dance, Fijian music and dance.

Clinical focus group 2024
Similar to the maternal focus groups conducted in August 2023, 10 staff of Community Health Centers, Inc. from clinicians, front desk staff, executive team members, and all levels of roles within the Federally Qualified Health Center (FQHC) participated in a focus group. Questions centered around barriers and potential solutions to improving maternal health outcomes for Community Health Centers, Inc. patients.
UWNQC produced an infographic representation of what was shared in the focus group, and overarching topics. UWNQC partners with Community Health Centers, Inc. on quality improvement work to respond to and aim to improve issues that were discussed in the focus group.
CHC Focus Group Summary May 2024 (PDF)
Maternal focus groups 2023
In August 2023, three focus groups were conducted to understand the experiences of and resources needed by Utah moms in the Hispanic/Latina, Native Hawaiian and Pacific Islander, and Black/African American communities to improve maternal health outcomes. This was a collaboration between Comunidad Materna, Utah Pacific Islander Health Coalition, and Project Success Coalition; the DHHS Utah Women and Newborns Quality Collaborative; and the DHHS Office of Health Equity. DHHS epidemiologists from the Office of Maternal and Child Health and the Office of Health Equity are currently working on qualitative data analysis.
These initial three infographic summaries serve as an overview of what was shared in these focus groups and as a way to share back the information with their communities of origin. Each focus group was led by a member of the respective community, and the findings were reviewed by members of each group before publishing. These focus groups were funded by the Centers for Disease Control and Prevention as part of a cooperative agreement with the goal of reducing maternal health disparities. See below for full PDFs.
English & Spanish - Hispanic Maternal Focus Group Summary 2023 (PDF)
The Maternal Resource Guide
The Improving Maternal Health Outcomes Committee has worked on a quality improvement project to develop a maternal resource guide. The guide provides info and over 900+ resources statewide on key social drivers of health, such as housing and food resources. This guide is in both English and Spanish.
Thank you to the committee and to Dr. Frank Powers for his leadership on this project.
The guide can be accessed at MaternalResources.Utah.gov

Mental health general resource flyer
This resource was created to share widely with the public, including urgent care centers.
Maternal mental health resource flyer
This resource was created to share widely with the public, including urgent care centers.